The amylase has a three-dimensional structure capable of binding to substrate and, by the action of highly specific catalytic groups, promote the breakage of the glycoside links.
The human alpha-amylase is a classical calcium-containing enzyme composed of 512 amino acids in a single oligosaccharide chain with a molecular weight of 57.6 kDa.
The main function of amylase is to hydrolyze the glycosidic bonds in starch molecules, converting complex carbohydrates to simple sugars.
Salivary amylase is the first step in the chemical digestion of food. This is one of the major reasons that it is so important for people to take time while eating and thoroughly chew their food. Once a food bolus is swallowed and infiltrated with gastric juice, its catabolic activity is mostly stopped by low acidic pH
As the starches, polysaccharides, and complex carbohydrates continue through the digestive tract, they are further broken down from additional amylase released from the pancreas into the proximal small intestine.
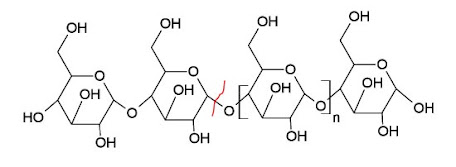
There are three main classes of amylase enzymes; Alpha-, beta- and gamma-amylase, and each act on different parts of the carbohydrate molecule. Alpha-amylase is widespread among living organisms. In the digestive systems of humans and many other mammals, an alpha-amylase called ptyalin is produced by the salivary glands. Alpha-amylase is an enzyme that catalyzes the hydrolysis of internal α-1,4-glycosidic linkages in starch and glycogen, yielding such glucose, maltose and maltotriose units.
Beta-amylase is found in microbes and plants. Gamma-amylase is found in animals and plants. This article will focus on alpha-amylase and its applications
Enzyme amylases
 |
| α-amylase |



