The term sugar describes the chemical class of carbohydrates (qv) of the general formula Cn(H2O)n-1 or (CH2O)n for monosaccharides. Sugar is a building blocks of carbohydrates and it is naturally found in many foods such as fruit, milk, vegetables and grain, another kind of sugar is added sugar which can be founded in flavored yogurt, sweetened beverages, baked goods and cereals, and it is used widely in industry.
To most people, “sweet” is synonymous with table sugar (sucrose), which is derived from sugarcane or sugar beets and contains 16 calories per teaspoon. Colloquially, sugar is the common name for sucrose, the solid crystalline sweetener for foods and beverages.
The sweet taste of sucrose is its most notable and important physical property and is regarded as the standard against which other sweeteners (qv) are rated. Sweetness is influenced by temperature, pH, sugar concentration, physical properties of the food system, and other factors.
Sucrose is a simple carbohydrate and occurs naturally in plants because they make sucrose via photosynthesis. The highest concentrations of sucrose are found in sugar cane and sugar beets, in amounts ranging from 12–15 and 13–20% by weight, respectively. Sugar cane and sugar beets are the main sources for making commercial sugar. .
Table sugar – sucrose
The primary goal of food is to promote our health and general well-being. Food science entails comprehending the characteristics, composition, and behaviors of food constituents in different situations, such as storage, handling, and consumption.
Showing posts with label sucrose. Show all posts
Showing posts with label sucrose. Show all posts
December 16, 2021
October 29, 2014
Table sugar of sucrose
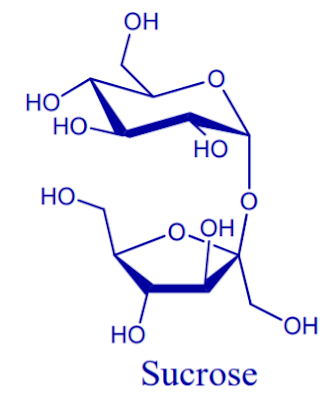
Cane and beet sugar are identified in technical terms as sucrose. It is composite molecule made of one molecule each of glucose and fructose.
Sucrose is one of the most abundant carbohydrates found in nature and is a major component of the food chain. Sucrose is a 12-carbon sugar that is broken down in the intestine to glucose and fructose, hence utilized as a source of energy.
The official name of sucrose, according to the IUPAC-IUB Commission of Biochemical nomenclatures is β–D-fructofuranosyl-α-D-glucopyranoside.
Solubility of sugars varies with sugar types. For example, sucrose is more soluble than glucose and less soluble than fructose. This influence candy types and product success. It is the second most soluble sugar – two parts can dissolve in one part of room temperature.
To increase the solubility of sucrose and reduce possible undesirable crystallization, sucrose may be treated by inversions become invert sugar. When a sucrose solution is heated with an acid, some of the sucrose breaks down into equal parts of two simple sugars, dextrose and levulose. A mixture of equal parts of dextrose and levulose is called invert sugar.
Table sugar of sucrose
Sucrose is one of the most abundant carbohydrates found in nature and is a major component of the food chain. Sucrose is a 12-carbon sugar that is broken down in the intestine to glucose and fructose, hence utilized as a source of energy.
The official name of sucrose, according to the IUPAC-IUB Commission of Biochemical nomenclatures is β–D-fructofuranosyl-α-D-glucopyranoside.
Solubility of sugars varies with sugar types. For example, sucrose is more soluble than glucose and less soluble than fructose. This influence candy types and product success. It is the second most soluble sugar – two parts can dissolve in one part of room temperature.
To increase the solubility of sucrose and reduce possible undesirable crystallization, sucrose may be treated by inversions become invert sugar. When a sucrose solution is heated with an acid, some of the sucrose breaks down into equal parts of two simple sugars, dextrose and levulose. A mixture of equal parts of dextrose and levulose is called invert sugar.
Table sugar of sucrose
March 13, 2012
Sucrose and sugar
Sucrose is a basic carbohydrate and has occupied a central position in human food for centuries. Sugar contributes to the pleasant taste and physical structure of many foods.
Sucrose is the scientific name for table sugar. It is a composite molecule mad of one molecule each of glucose and fructose.
Sucrose is known under many trade and popular names. This may may be related to its purity grade to its extent of granulation or crystal size and to its use.
All sugars are members of the larger group of compounds called carbohydrates and are characterized by a sweet taste.
The sucrose available in the markets as sugar has been extracted from sugar cane or sugar beet, but sucrose is also abundant in most plant materials , particularly fruit.
Sugar, in its pure state it is normally available as white crystals, but it can also be bought as liquid sugar, which is a solution in water.
Impure sucrose, crystals with coatings of syrup which are dark in color, are known as ‘brown sugar’.
Sucrose is a disaccharides and a non reducing sugar.
When a solution of sucrose is heated in the presence of some acid, it breaks apart into its tow sugars. Certain sucrose into glucose and fructose is often referred it as’ inversion’ and the resulting mixture is called ‘invert sugar’ or ‘invert syrup.’
Sucrose is more soluble than glucose and less soluble than fructose. In its dried, granular form, sugar become increasingly soluble in water with an increase in temperature.
Sugar may precipitate from solution, forming an undesirable grainy, crystalline product.
Sucrose and sugar
Sucrose is the scientific name for table sugar. It is a composite molecule mad of one molecule each of glucose and fructose.
Sucrose is known under many trade and popular names. This may may be related to its purity grade to its extent of granulation or crystal size and to its use.
All sugars are members of the larger group of compounds called carbohydrates and are characterized by a sweet taste.
The sucrose available in the markets as sugar has been extracted from sugar cane or sugar beet, but sucrose is also abundant in most plant materials , particularly fruit.
Sugar, in its pure state it is normally available as white crystals, but it can also be bought as liquid sugar, which is a solution in water.
Impure sucrose, crystals with coatings of syrup which are dark in color, are known as ‘brown sugar’.
Sucrose is a disaccharides and a non reducing sugar.
When a solution of sucrose is heated in the presence of some acid, it breaks apart into its tow sugars. Certain sucrose into glucose and fructose is often referred it as’ inversion’ and the resulting mixture is called ‘invert sugar’ or ‘invert syrup.’
Sucrose is more soluble than glucose and less soluble than fructose. In its dried, granular form, sugar become increasingly soluble in water with an increase in temperature.
Sugar may precipitate from solution, forming an undesirable grainy, crystalline product.
Sucrose and sugar
November 4, 2011
Sucrose
Three disaccharides are important in nutrition: maltose, sucrose and lactose. All three have glucose as one of their single sugars.
Sucrose is the most common disaccharide and it contains glucose and fructose joined together by an alpha-1,2-glyccosidic link.
Sucrose is the chemical name for what is commonly called white sugar, table sugar, granulated sugar or simply sugar. Sucrose provides some of the natural sweetness, of honey, maple syrup, fruits and vegetables.
The carbonyl groups of both the glucose and the fructose molecule are involved in the glycosidic bond; thus, the configuration of each monosaccharide become fixed.
Sucrose can be hydrolyzed to glucose and fructose by heat and acid or by the enzymes invertase or sucrose.
Sucrose is found in many plants and is especially abundant in sugar cane and sugar beets. These plants can be crushed to produce a juice that is recessed to make a brown liquid called molasses.
The equimolar mixture of glucose and the fructose produced in this way is called invert sugar.
Production of inverts sugar is important during the formation of candies and jellies, as inverts sugar prevents unwanted or excessive crystallization of sucrose.
Manufacturers use a refining process to extract sucrose from the juices of sugar cane or sugar beets. Full refining removes impurities white sugar and powdered sugar are so highly refined that they are virtually 100 percent sucrose.
Sucrose
Sucrose is the most common disaccharide and it contains glucose and fructose joined together by an alpha-1,2-glyccosidic link.
Sucrose is the chemical name for what is commonly called white sugar, table sugar, granulated sugar or simply sugar. Sucrose provides some of the natural sweetness, of honey, maple syrup, fruits and vegetables.
The carbonyl groups of both the glucose and the fructose molecule are involved in the glycosidic bond; thus, the configuration of each monosaccharide become fixed.
Sucrose can be hydrolyzed to glucose and fructose by heat and acid or by the enzymes invertase or sucrose.
Sucrose is found in many plants and is especially abundant in sugar cane and sugar beets. These plants can be crushed to produce a juice that is recessed to make a brown liquid called molasses.
The equimolar mixture of glucose and the fructose produced in this way is called invert sugar.
Production of inverts sugar is important during the formation of candies and jellies, as inverts sugar prevents unwanted or excessive crystallization of sucrose.
Manufacturers use a refining process to extract sucrose from the juices of sugar cane or sugar beets. Full refining removes impurities white sugar and powdered sugar are so highly refined that they are virtually 100 percent sucrose.
Sucrose
August 17, 2011
Sugar processing
After harvesting, the cane goes through a series of processing steps for conversion to the final sugar product.
It is first washed to remove dirt and trash; then cut cane is chopped to remove the top section which has low sugar content, the leaves are removed and the remaining stem is chopped into short lengths for crushing.
Cane entering the mill is prepared by chopping, shredding or crushing and after one or a combination of these procedures is completed, the remaining juice is extracted by passing the prepared cane though series of mills containing three to five rollers.
The other method of extracting juice is by diffusion where the sugar is leached out by water and thin juices.
The raw cane use contains soluble solids other than sucrose and is clarified before concentration, a very important step in facilitating subsequent recovery of the sucrose.
To remove both insoluble and the soluble impurities from the juice, the juice is limed, heated and sent to clarifiers.
Clarification and neutralization of the mildly acidic, raw extract (pH 4.8-5.0) is done by treatment with lime or lime and carbon dioxide.
The juice is heated to approximately 100 degree C which coagulates suspended solids and proteins, inactivates enzymes systems such as invertase and destroys bacteria, thus stabilizing the system.
The clarified juice, which contains only 12 to 13 % of sucrose, is sent to evaporators, while the juice containing precipitate is sent to rotary-drum vacuum filters to extract the juice leaving the filter cake for disposal.
The syrup is used to produce crystals (raw sugar) and syrup (molasses). The whole process is energy self sufficient as the remaining cane fiber is used to fire the boilers.
The raw sugar is shipped from the mill to a refinery, where it is decolorized with activated charcoal, or put through a column beef bone.
After being crystallized and dried the end product of sugar refining is white and nearly pure, sucrose.
Sugar processing
It is first washed to remove dirt and trash; then cut cane is chopped to remove the top section which has low sugar content, the leaves are removed and the remaining stem is chopped into short lengths for crushing.
Cane entering the mill is prepared by chopping, shredding or crushing and after one or a combination of these procedures is completed, the remaining juice is extracted by passing the prepared cane though series of mills containing three to five rollers.
The other method of extracting juice is by diffusion where the sugar is leached out by water and thin juices.
The raw cane use contains soluble solids other than sucrose and is clarified before concentration, a very important step in facilitating subsequent recovery of the sucrose.
To remove both insoluble and the soluble impurities from the juice, the juice is limed, heated and sent to clarifiers.
Clarification and neutralization of the mildly acidic, raw extract (pH 4.8-5.0) is done by treatment with lime or lime and carbon dioxide.
The juice is heated to approximately 100 degree C which coagulates suspended solids and proteins, inactivates enzymes systems such as invertase and destroys bacteria, thus stabilizing the system.
The clarified juice, which contains only 12 to 13 % of sucrose, is sent to evaporators, while the juice containing precipitate is sent to rotary-drum vacuum filters to extract the juice leaving the filter cake for disposal.
The syrup is used to produce crystals (raw sugar) and syrup (molasses). The whole process is energy self sufficient as the remaining cane fiber is used to fire the boilers.
The raw sugar is shipped from the mill to a refinery, where it is decolorized with activated charcoal, or put through a column beef bone.
After being crystallized and dried the end product of sugar refining is white and nearly pure, sucrose.
Sugar processing
May 13, 2007
Sugars

In United States, the natural sugars of milk, fruits, vegetables, and grains account for about half of the sugar intake, the other half consists of concentrated sugars that have been refined and added to foods for a variety of purposes.
Sugars, important in nutrition, consist of monosaccharides, having the general formula C6H12O6, and disaccharides, having the general formula C12H22O11.
Although the monosaccharides consists of 3-carbon sugars (trioses), 4-carbon sugars (tetroses), 5-carbon sugars (pentoses), and 6-carbon sugars (hexoses), only the latter are important in human nutrition as sources of energy.
Three monosaccharides are important in nutrition: glucose, fructose and galactose. All three monosaccharides have the same number and kinds of atoms but in different arrangements.
Glucose, a 6-carbon sugar, is one of the simplest carbohydrates found in foods. While many foods contain traces of glucose, it is found in significant amounts only in fruits, such as grapes.
Most cells depend on glucose for their fuel to some extent and the cells of the brain and the rest of the nervous system depend almost exclusively on glucose for their energy.
 Fructose, also a 6-carbon sugar, is found in ripened fruits and honey, both of these sugars can be utilizes by body as a source of energy. It is the sweetest of the natural sugars.
Fructose, also a 6-carbon sugar, is found in ripened fruits and honey, both of these sugars can be utilizes by body as a source of energy. It is the sweetest of the natural sugars.
Other source of fructose include soft drinks, ready to eat cereals and other products sweetened with high fructose corn syrup.
Lactose, the 12-carbon sugar present in milk, is broken down in the intestine to glucose and lactose (6-carbon sugar), both of which can be used as sources of energy.
Maltose, another disaccharide, produced form starch in the malting of grains is much less effective sweetener than sucrose.
The used of added sugars had risen steadily over the past several decades, both in the United States and around the world, with soft drinks and sugared fruit drinks accounting for most of the increase.
Sugars
Subscribe to:
Posts (Atom)
The Most Popular Posts
-
Crude fat is the term used to refer to the crude mixture of fat-soluble material present in a sample. Crude fat also known as the ether ext...
-
Ash or mineral content is the portion of the food or any organic material that remains after it is burned at very high temperatures. The a...
-
Crude fiber is a measure of the quantity of indigestible cellulose, pentosans, lignin, and other components of this type in present foods. ...
-
Density is the weight of a substance per unit of volume, while specific gravity is the ratio between the density of the substance and that o...
-
Gelatinization occurs when starch granules are heated in a liquid. It is responsible for the thickening of food systems. The process is an i...