Sugars play a crucial role in the American diet, with natural sugars from milk, fruits, vegetables, and grains constituting approximately half of our sugar intake. The other half comprises refined and added sugars, which are incorporated into various foods for different purposes.
In nutrition, sugars are classified into monosaccharides and disaccharides. Monosaccharides, with the chemical formula C6H12O6, and disaccharides, with the formula C12H22O11, are essential components of our diet.
Among monosaccharides, the 6-carbon sugars, known as hexoses, are particularly significant as energy sources for humans. Three key monosaccharides—glucose, fructose, and galactose—are vital for nutrition, each with distinct arrangements of atoms.
Glucose, a fundamental 6-carbon sugar, is primarily found in fruits like grapes. It serves as a crucial energy source for cells throughout the body, especially the brain and nervous system.
Fructose, also a 6-carbon sugar, is the sweetest natural sugar and is abundant in ripened fruits and honey. Additionally, it is a common ingredient in processed foods, including soft drinks and cereals, often in the form of high fructose corn syrup.
Lactose, a disaccharide found in milk, breaks down into glucose and galactose in the intestine, providing valuable energy sources for the body.
Maltose, another disaccharide derived from the malting of grains, is less commonly used as a sweetener compared to sucrose.
The consumption of added sugars has surged in recent decades, both in the United States and globally. Soft drinks and sugary fruit beverages are major contributors to this increase, leading to concerns about their impact on health.
In conclusion, understanding the different types of sugars and their sources is essential for maintaining a balanced diet. While natural sugars provide valuable nutrients, excessive consumption of added sugars can contribute to various health issues. Thus, it's crucial to make informed choices about sugar intake to promote overall well-being.
Understanding Sugars and Their Impact on Nutrition
The primary goal of food is to promote our health and general well-being. Food science entails comprehending the characteristics, composition, and behaviors of food constituents in different situations, such as storage, handling, and consumption.
Showing posts with label sugar. Show all posts
Showing posts with label sugar. Show all posts
April 19, 2024
April 5, 2024
The Versatile Role of Sugar as a Preservative
Sugar, beyond its delightful sweetness, serves as a multifaceted ingredient in the culinary world. From the delectable allure of cookies to the indulgent richness of chocolate, sugar plays a pivotal role not only in enhancing flavors but also in preserving various food products.
At its core, sugar, in its pure crystalline or powdered form, predominantly derived from sugar cane and sugar beets, embodies the essence of sweetness. However, its significance transcends mere taste; it stands as one of the most widely used preservatives in the culinary realm.
The preservation of fruit products through sugar encompasses two distinct processes: simultaneous concentration and simple addition. Jams and marmalades exemplify the former, where the synergy of sugar and fruit undergoes a transformative process, culminating in delectable spreads that boast extended shelf life. Conversely, the addition of sugar without concentration finds its application in preserving delicacies like candied fruit, lemon peel, and orange peel, preserving their essence and texture for prolonged enjoyment.
Moreover, sugar's prowess extends to the realm of fruit juices, where it acts as a potent preservative. Through the elevation of sugar content via either bulk addition or evaporation-induced concentration, fruit juices transform into enduring syrups and concentrates, prolonging their freshness and flavor.
Central to sugar's preservation capabilities lies its composition, predominantly comprising fructose, sucrose, and lactose. While sucrose enriches baked goods with its innate sweetness, it concurrently serves as a linchpin in their preservation. Beyond baked treats, sucrose assumes the mantle of a preservative in a myriad of confections, including marzipan, nougat, chocolate, and pralines, safeguarding their integrity and enhancing their longevity.
Moreover, the latest advancements in food science continue to underscore sugar's efficacy as a preservative. Innovations in sugar-based preservation techniques not only extend the shelf life of perishable goods but also cater to evolving consumer preferences for natural and familiar ingredients.
In essence, sugar emerges not only as a quintessential element in crafting irresistible culinary delights but also as a stalwart guardian of freshness and flavor. Its enduring legacy in the realm of preservation underscores its timeless relevance in the ever-evolving landscape of gastronomy.
The Versatile Role of Sugar as a Preservative
At its core, sugar, in its pure crystalline or powdered form, predominantly derived from sugar cane and sugar beets, embodies the essence of sweetness. However, its significance transcends mere taste; it stands as one of the most widely used preservatives in the culinary realm.
The preservation of fruit products through sugar encompasses two distinct processes: simultaneous concentration and simple addition. Jams and marmalades exemplify the former, where the synergy of sugar and fruit undergoes a transformative process, culminating in delectable spreads that boast extended shelf life. Conversely, the addition of sugar without concentration finds its application in preserving delicacies like candied fruit, lemon peel, and orange peel, preserving their essence and texture for prolonged enjoyment.
Moreover, sugar's prowess extends to the realm of fruit juices, where it acts as a potent preservative. Through the elevation of sugar content via either bulk addition or evaporation-induced concentration, fruit juices transform into enduring syrups and concentrates, prolonging their freshness and flavor.
Central to sugar's preservation capabilities lies its composition, predominantly comprising fructose, sucrose, and lactose. While sucrose enriches baked goods with its innate sweetness, it concurrently serves as a linchpin in their preservation. Beyond baked treats, sucrose assumes the mantle of a preservative in a myriad of confections, including marzipan, nougat, chocolate, and pralines, safeguarding their integrity and enhancing their longevity.
Moreover, the latest advancements in food science continue to underscore sugar's efficacy as a preservative. Innovations in sugar-based preservation techniques not only extend the shelf life of perishable goods but also cater to evolving consumer preferences for natural and familiar ingredients.
In essence, sugar emerges not only as a quintessential element in crafting irresistible culinary delights but also as a stalwart guardian of freshness and flavor. Its enduring legacy in the realm of preservation underscores its timeless relevance in the ever-evolving landscape of gastronomy.
The Versatile Role of Sugar as a Preservative
December 16, 2021
Table sugar – sucrose
The term sugar describes the chemical class of carbohydrates (qv) of the general formula Cn(H2O)n-1 or (CH2O)n for monosaccharides. Sugar is a building blocks of carbohydrates and it is naturally found in many foods such as fruit, milk, vegetables and grain, another kind of sugar is added sugar which can be founded in flavored yogurt, sweetened beverages, baked goods and cereals, and it is used widely in industry.
To most people, “sweet” is synonymous with table sugar (sucrose), which is derived from sugarcane or sugar beets and contains 16 calories per teaspoon. Colloquially, sugar is the common name for sucrose, the solid crystalline sweetener for foods and beverages.
The sweet taste of sucrose is its most notable and important physical property and is regarded as the standard against which other sweeteners (qv) are rated. Sweetness is influenced by temperature, pH, sugar concentration, physical properties of the food system, and other factors.
Sucrose is a simple carbohydrate and occurs naturally in plants because they make sucrose via photosynthesis. The highest concentrations of sucrose are found in sugar cane and sugar beets, in amounts ranging from 12–15 and 13–20% by weight, respectively. Sugar cane and sugar beets are the main sources for making commercial sugar. .
Table sugar – sucrose
To most people, “sweet” is synonymous with table sugar (sucrose), which is derived from sugarcane or sugar beets and contains 16 calories per teaspoon. Colloquially, sugar is the common name for sucrose, the solid crystalline sweetener for foods and beverages.
The sweet taste of sucrose is its most notable and important physical property and is regarded as the standard against which other sweeteners (qv) are rated. Sweetness is influenced by temperature, pH, sugar concentration, physical properties of the food system, and other factors.
Sucrose is a simple carbohydrate and occurs naturally in plants because they make sucrose via photosynthesis. The highest concentrations of sucrose are found in sugar cane and sugar beets, in amounts ranging from 12–15 and 13–20% by weight, respectively. Sugar cane and sugar beets are the main sources for making commercial sugar. .
Table sugar – sucrose
October 4, 2021
Main roles of sugar in food
Sucrose, glucose and fructose are the most common sweeteners in nature.
Glucose is always less sweet than sucrose, whereas the sweetness of
fructose is highly dependent on temperature.
Sugar, which refers usually to sucrose, is natural and nontoxic, sweet testing, water soluble crystalline carbohydrates, and every 1 gram of sugar provide body 4K.calories. The main source for sugar is the beet sugar or cane sugar; also there are several sources such as honey, corn syrup, fruits, and vegetables….etc. Sucrose provides a sweetness flavour profile which is consistently liked by consumers at an economical cost.
The relatively high solubility of sucrose is an important parameter for its bulking effect in many foods and beverages. The dissolved sugar increases the viscosity of water-based solutions or mixtures, resulting in enhanced mouthfeel. Dissolved sugar lowers the freezing point of ice cream by preventing the water molecules from combining to form ice crystals, which slows down the freezing process.
By absorbing free water and increasing osmotic pressure, sugar reduces water activity in a food system (e.g. jam), resulting in reduced microbial and mold growth as well as extending the storage life of food. Also sugar can preserve fruits, either in syrup with fruit such as apples, pears.
Crystallization of sugars is desirable in products such as fondant, dragees, fudge etc., but not in many other products like jam and jellies. Crystallization occurs when the solubility limit of the sugar, typically sucrose or glucose, has been exceeded and a supersaturated environment has been created.
Sugar plays an important and single role in contributing to the flavor of food by interacting with other components to enhance or lessen certain flavors. By adding a small amount of sugar to cooked vegetables and meat enhance the food’s natural flavors, without making them taste sweet.
Texture is an expression of the sensation in the mouth. Sugar affects this by providing volume and consistency in many products such as bread, jam and beverages.In bread, sugar affects the volume of dough by speeding up the fermentation process. This gives the bread a more porous structure and softer crumb.
Main roles of sugar in food
Sugar, which refers usually to sucrose, is natural and nontoxic, sweet testing, water soluble crystalline carbohydrates, and every 1 gram of sugar provide body 4K.calories. The main source for sugar is the beet sugar or cane sugar; also there are several sources such as honey, corn syrup, fruits, and vegetables….etc. Sucrose provides a sweetness flavour profile which is consistently liked by consumers at an economical cost.
The relatively high solubility of sucrose is an important parameter for its bulking effect in many foods and beverages. The dissolved sugar increases the viscosity of water-based solutions or mixtures, resulting in enhanced mouthfeel. Dissolved sugar lowers the freezing point of ice cream by preventing the water molecules from combining to form ice crystals, which slows down the freezing process.
By absorbing free water and increasing osmotic pressure, sugar reduces water activity in a food system (e.g. jam), resulting in reduced microbial and mold growth as well as extending the storage life of food. Also sugar can preserve fruits, either in syrup with fruit such as apples, pears.
Crystallization of sugars is desirable in products such as fondant, dragees, fudge etc., but not in many other products like jam and jellies. Crystallization occurs when the solubility limit of the sugar, typically sucrose or glucose, has been exceeded and a supersaturated environment has been created.
Sugar plays an important and single role in contributing to the flavor of food by interacting with other components to enhance or lessen certain flavors. By adding a small amount of sugar to cooked vegetables and meat enhance the food’s natural flavors, without making them taste sweet.
Texture is an expression of the sensation in the mouth. Sugar affects this by providing volume and consistency in many products such as bread, jam and beverages.In bread, sugar affects the volume of dough by speeding up the fermentation process. This gives the bread a more porous structure and softer crumb.
Main roles of sugar in food
August 8, 2021
History and discovery of glucose
A sugar industry existed around the shores of the Mediterranean between A.D. 700-1600. It was founded as part of the Arab agricultural revolution. The first reference of glucose was “grape sugar” in Moorish writing 1100 AD. The Moors introduced sugar to Europe for the first time by bringing sugarcane from the Nile valley. It grew particularly well along the Mediterranean coast and especially in the Málaga area of al-Andalus.
The sweetness of sugarbeets was recorded in 1590 AD. In 1600, the French agronomist Olivier de Serres noted that ‘‘The beet on being cooked yields a syrup which is beautiful to look at on account of its vermillion color’’.
Glucose, a ubiquitous carbon source preferred by most cells, was first identified by German pharmacist, Andreas Marggraf in 1747. In that year he used alcohol to isolated sucrose from sugar beets, arguably his most influential discovery, as it has revolutionized the modern sugar industry with the process, he used to extract such sugar. He identified the sugar beet’s dried, crystallized juice as identical with cane sugar by the use of a microscope.
In the same year, he experimented with raisins to extract glucose. Raisins are comprised of many molecules, including many sugars like sucrose, fructose, and glucose.
The name glucose was coined in 1838 by French chemist Jean Dumas, from the Greek word gleucos, which means ‘sweet’ or ‘sugar,’ and the structure was discovered by Emil Fischer around the turn of the century.
In 1884, Emil Fischer synthesized some of the known sugars such as fructose and glucose, and he identified 16 stereoisomeric forms of glucose.
History and discovery of glucose
The sweetness of sugarbeets was recorded in 1590 AD. In 1600, the French agronomist Olivier de Serres noted that ‘‘The beet on being cooked yields a syrup which is beautiful to look at on account of its vermillion color’’.
Glucose, a ubiquitous carbon source preferred by most cells, was first identified by German pharmacist, Andreas Marggraf in 1747. In that year he used alcohol to isolated sucrose from sugar beets, arguably his most influential discovery, as it has revolutionized the modern sugar industry with the process, he used to extract such sugar. He identified the sugar beet’s dried, crystallized juice as identical with cane sugar by the use of a microscope.
In the same year, he experimented with raisins to extract glucose. Raisins are comprised of many molecules, including many sugars like sucrose, fructose, and glucose.
The name glucose was coined in 1838 by French chemist Jean Dumas, from the Greek word gleucos, which means ‘sweet’ or ‘sugar,’ and the structure was discovered by Emil Fischer around the turn of the century.
In 1884, Emil Fischer synthesized some of the known sugars such as fructose and glucose, and he identified 16 stereoisomeric forms of glucose.
History and discovery of glucose
June 25, 2020
Sugarcane
Sugarcane is a large grass of the genus Saccharum, tribe Andropogoneae, family Poaceae. Modern sugarcane (Saccharum spp.) cultivars are interspecific hybrids derived from a hybridization process involving Saccharum officinarum (or “noble cane”) and Saccharum spontaneum (wild cane), followed by a series of backcrosses to the noble parent.
Sugarcane is a highly productive C4 grass used as the main source of sugar and more recently to produce ethanol, a renewable transportation fuel. There is increased interest in this crop due to the impending need to decrease the dependency on fossil fuels.
Initially, pieces of cane stalk would have been chewed to express the sweet juice, and chewing canes still provide a conveniently packaged form of energy food in many cultures. Juice extraction from the stalk, and concentrating it by drying or heating to produce a crude sugary product, must have been developed in a rudimentary form at least 3000 years ago.
Sugarcane is a tall perennial tropical grass, which tillers at the base to produce unbranched stems from 2 to 4 m or more tall, and to around 5 cm in diameter. It is cultivated for these thick stems, stalks or canes, from which the sugar is extracted.
Sugarcane is cultivated for its high rate of sucrose accumulation, ease of propagation via vegetative stem cuttings and multiple harvests from a single planting. It is a principal crop in tropical and subtropical regions, with a production estimate of over 1.3 million metric tons of sucrose per annum.
Sugarcane is the main source of sugar (80%) globally. The sugar juice is used for making white sugar, brown sugar and jaggery. The main by-products of sugarcane industry are bagasse and molasses. Bagasse is mainly used as fuel. It is also used for production of compressed fibre board paper, plastic and others. Molasses is used in distilleries for the manufacturing of ethyl alcohol, butyl alcohol, citric acid etc. Rum is the best potable spirit made from molasses.
The ideal environment for sugarcane is one in which rainfall (or irrigation) is well distributed during the growing season, but where the preharvest ripening period is relatively dry, and the sunshine hours are plentiful throughout the whole season.
Sugarcane
Sugarcane is a highly productive C4 grass used as the main source of sugar and more recently to produce ethanol, a renewable transportation fuel. There is increased interest in this crop due to the impending need to decrease the dependency on fossil fuels.
Initially, pieces of cane stalk would have been chewed to express the sweet juice, and chewing canes still provide a conveniently packaged form of energy food in many cultures. Juice extraction from the stalk, and concentrating it by drying or heating to produce a crude sugary product, must have been developed in a rudimentary form at least 3000 years ago.
Sugarcane is a tall perennial tropical grass, which tillers at the base to produce unbranched stems from 2 to 4 m or more tall, and to around 5 cm in diameter. It is cultivated for these thick stems, stalks or canes, from which the sugar is extracted.
Sugarcane is cultivated for its high rate of sucrose accumulation, ease of propagation via vegetative stem cuttings and multiple harvests from a single planting. It is a principal crop in tropical and subtropical regions, with a production estimate of over 1.3 million metric tons of sucrose per annum.
Sugarcane is the main source of sugar (80%) globally. The sugar juice is used for making white sugar, brown sugar and jaggery. The main by-products of sugarcane industry are bagasse and molasses. Bagasse is mainly used as fuel. It is also used for production of compressed fibre board paper, plastic and others. Molasses is used in distilleries for the manufacturing of ethyl alcohol, butyl alcohol, citric acid etc. Rum is the best potable spirit made from molasses.
The ideal environment for sugarcane is one in which rainfall (or irrigation) is well distributed during the growing season, but where the preharvest ripening period is relatively dry, and the sunshine hours are plentiful throughout the whole season.
Sugarcane
August 27, 2016
Simple sugars or monosaccharides
The two main types of sugars are monosaccharides and disaccharides. Monosaccharides consist of a single sugar molecule.
Monosaccharides may contain from three to nine carbon atoms although most of them contain five or six. A three carbon monosaccharide is called a triose; one containing four carbons is called a tetrose; five, a pentose; six, a hexose; seven, a heptose; eight an octose and nine a nonose.
*Pentoses (Arabinose, ribose, xylose)
The pentose sugars, deoxyribose and ribose, are essential components of the genetic material DNA and RNA. E.g. glucose and fructose: (C6H12O6); arabinose and xylose: (C5H10O5).
Arabinose is found in gums and when several arabinose molecules are joined together, a pentosan formed.
*Hexoses
-Aldohexoses – galactose, glucose
-Ketohexose - fructose
All hexoses have the same chemical formula C6H12O6, but slightly different structures. In nature, only fructose and glucose occur in free form. Galactose joined with glucose forms the disaccharide lactose.
Glucose is a hexose. Octoses and nanoses are quite rare. Glucose, the main source of energy for body cells, is found in most sweet fruits and in blood.
Simple sugars or monosaccharides
Monosaccharides may contain from three to nine carbon atoms although most of them contain five or six. A three carbon monosaccharide is called a triose; one containing four carbons is called a tetrose; five, a pentose; six, a hexose; seven, a heptose; eight an octose and nine a nonose.
*Pentoses (Arabinose, ribose, xylose)
The pentose sugars, deoxyribose and ribose, are essential components of the genetic material DNA and RNA. E.g. glucose and fructose: (C6H12O6); arabinose and xylose: (C5H10O5).
Arabinose is found in gums and when several arabinose molecules are joined together, a pentosan formed.
*Hexoses
-Aldohexoses – galactose, glucose
-Ketohexose - fructose
All hexoses have the same chemical formula C6H12O6, but slightly different structures. In nature, only fructose and glucose occur in free form. Galactose joined with glucose forms the disaccharide lactose.
Glucose is a hexose. Octoses and nanoses are quite rare. Glucose, the main source of energy for body cells, is found in most sweet fruits and in blood.
Simple sugars or monosaccharides
November 24, 2014
Complex carbohydrate of oligosaccharide
Complex carbohydrates consist of many monosaccharides bonded together in a variety of bonding patterns.
Oligosaccharides yield 3 to 6 monosaccharide units on hydrolysis. These carbohydrates are attached to either the side chain oxygen atom of serine or threonine residues by O-glycosidic linkages or to the side chain nitrogen of asparagine residues by N-glycosidic linkages.
The commonly found oligosaccharides in foods are sucrose, maltose, lactose, raffinose and stachyose.
Sucrose found throughout the plant world is most abundant in sugarcane, sugar beet and maple syrup. It is the familiar table sugar.
Foods high in oligosaccharides include onions, chicory, Jerusalem artichoke, asparagus globe artichoke, leek, garlic banana, and wheat. Oligosaccharides also present in dried beans, soybeans, peas and lentils.
Oligosaccharides have been dietary staples since antiquity and have received mush less attention than other carbohydrates, including simple sugars or dietary fiber.
In the body, oligosaccharides are components of cell membranes allowing cells to recognized and interact with one another.
Oligosaccharides play a key role in processes that take place in the surfaces of cells, particularly in cell-cell interactions and immune recognition.
Lately, interest in oligosaccharides has increased duetheir functional properties. These induce sweetening, ability, fat replacement, and enhancement of a ‘healthy’ gastrointestinal tract.
Complex carbohydrate of oligosaccharide
Oligosaccharides yield 3 to 6 monosaccharide units on hydrolysis. These carbohydrates are attached to either the side chain oxygen atom of serine or threonine residues by O-glycosidic linkages or to the side chain nitrogen of asparagine residues by N-glycosidic linkages.
The commonly found oligosaccharides in foods are sucrose, maltose, lactose, raffinose and stachyose.
Sucrose found throughout the plant world is most abundant in sugarcane, sugar beet and maple syrup. It is the familiar table sugar.
Foods high in oligosaccharides include onions, chicory, Jerusalem artichoke, asparagus globe artichoke, leek, garlic banana, and wheat. Oligosaccharides also present in dried beans, soybeans, peas and lentils.
Oligosaccharides have been dietary staples since antiquity and have received mush less attention than other carbohydrates, including simple sugars or dietary fiber.
In the body, oligosaccharides are components of cell membranes allowing cells to recognized and interact with one another.
Oligosaccharides play a key role in processes that take place in the surfaces of cells, particularly in cell-cell interactions and immune recognition.
Lately, interest in oligosaccharides has increased duetheir functional properties. These induce sweetening, ability, fat replacement, and enhancement of a ‘healthy’ gastrointestinal tract.
Complex carbohydrate of oligosaccharide
October 29, 2014
Table sugar of sucrose
Cane and beet sugar are identified in technical terms as sucrose. It is composite molecule made of one molecule each of glucose and fructose.
Sucrose is one of the most abundant carbohydrates found in nature and is a major component of the food chain. Sucrose is a 12-carbon sugar that is broken down in the intestine to glucose and fructose, hence utilized as a source of energy.
The official name of sucrose, according to the IUPAC-IUB Commission of Biochemical nomenclatures is β–D-fructofuranosyl-α-D-glucopyranoside.
Solubility of sugars varies with sugar types. For example, sucrose is more soluble than glucose and less soluble than fructose. This influence candy types and product success. It is the second most soluble sugar – two parts can dissolve in one part of room temperature.
To increase the solubility of sucrose and reduce possible undesirable crystallization, sucrose may be treated by inversions become invert sugar. When a sucrose solution is heated with an acid, some of the sucrose breaks down into equal parts of two simple sugars, dextrose and levulose. A mixture of equal parts of dextrose and levulose is called invert sugar.
Table sugar of sucrose
Sucrose is one of the most abundant carbohydrates found in nature and is a major component of the food chain. Sucrose is a 12-carbon sugar that is broken down in the intestine to glucose and fructose, hence utilized as a source of energy.
The official name of sucrose, according to the IUPAC-IUB Commission of Biochemical nomenclatures is β–D-fructofuranosyl-α-D-glucopyranoside.
Solubility of sugars varies with sugar types. For example, sucrose is more soluble than glucose and less soluble than fructose. This influence candy types and product success. It is the second most soluble sugar – two parts can dissolve in one part of room temperature.
To increase the solubility of sucrose and reduce possible undesirable crystallization, sucrose may be treated by inversions become invert sugar. When a sucrose solution is heated with an acid, some of the sucrose breaks down into equal parts of two simple sugars, dextrose and levulose. A mixture of equal parts of dextrose and levulose is called invert sugar.
Table sugar of sucrose
September 16, 2014
What are monosaccharide sugars?
The term ‘monosaccharide’ refers to simple sugars containing up to ten carbon atoms per molecule. Monosaccharides are the simplest carbohydrates of one unit, so they cannot be further decomposed to a simpler sugar.
They are simply known as sugars. Same sugars occur in nature and others are synthetic.
Monosaccharides are single-sugar molecules that are made up of carbon, hydrogen and oxygen atoms in the ration 1:2:1.
A hydrolysis reaction separates disaccharides into monosaccharides. During hydrolysis, the addition of molecule water splits the bond between the two sugar molecules, providing the H and OH groups necessary for the sugars to exist as monosaccharaides.
Monosaccharides are the simplest form of carbohydrates. They are sweet, require no digestion, and can be absorbed directly into the bloodstream from the small intestine. They include glucose, fructose, and galactose. Although the structures of these sugars differ, they all have one thing in common: each contains six carbons atoms.
Glucose, also called dextrose, is the form of carbohydrate to which all other forms are converted for eventual metabolism. Glucose is one of the most abundant organic compounds on earth. Glucose exists in some fruits such as grapes, figs, and dates.
Fructose also called levulose or fruit sugar is found with glucose in many fruits and in honey. Industrially, it can be produced from sucrose or inulin.
Galactose is a product of the digestion of milk. It is one of two single sugars that are bound together to make up the sugar of milk.
What are monosaccharide sugars?
They are simply known as sugars. Same sugars occur in nature and others are synthetic.
Monosaccharides are single-sugar molecules that are made up of carbon, hydrogen and oxygen atoms in the ration 1:2:1.
A hydrolysis reaction separates disaccharides into monosaccharides. During hydrolysis, the addition of molecule water splits the bond between the two sugar molecules, providing the H and OH groups necessary for the sugars to exist as monosaccharaides.
Monosaccharides are the simplest form of carbohydrates. They are sweet, require no digestion, and can be absorbed directly into the bloodstream from the small intestine. They include glucose, fructose, and galactose. Although the structures of these sugars differ, they all have one thing in common: each contains six carbons atoms.
Glucose, also called dextrose, is the form of carbohydrate to which all other forms are converted for eventual metabolism. Glucose is one of the most abundant organic compounds on earth. Glucose exists in some fruits such as grapes, figs, and dates.
Fructose also called levulose or fruit sugar is found with glucose in many fruits and in honey. Industrially, it can be produced from sucrose or inulin.
Galactose is a product of the digestion of milk. It is one of two single sugars that are bound together to make up the sugar of milk.
What are monosaccharide sugars?
April 24, 2014
Caramel as food additive
Caramel is a complex mixture of brown flavoring/coloring substances produced when sugars are heated above their melting point during caramelization. During heating, the compounds breakdown and reassemble to form hundreds of different molecules that add flavor and aroma to foods.
Caramel is also used in flavorings and flavor enhancers for a wide range of foods, including caramels, cakes, and biscuits.
There are four classes of caramel used as food additives and they are defined by the reactant added to the carbohydrate during production.
*Plain caramel
*Caustic caramel
*Ammonia caramel
*Sulfite ammonia caramel
Caramel colorant must be compatible with food products in which they are used, which usually means the absence of flocculation and precipitation in the food.
Caramel is made up from the following food-grade carbohydrate: dextrose, invert sugar, lactose, malt syrup, molasses, starch hydrolysates, and fraction thereof and sucrose, by carefully controlled heat treatment. A large amount of commercial caramel is produced from liquid corn syrup or glucose syrup.
Caramel coloring is freely soluble in water and insoluble in most organic solvents. In concentrated form the colorant has a distinctive burned taste that is unnoticeable at the typical levels of use.
Caramel colors are the most widely used food coloring agents, contributing about 90% by weight of the total coloring agents supplied in the UK food industry. World-wide 80% has been quoted.
Caramelization is done in the industry with different catalysts to produce either flavor or color.
For flavor purposes, sucrose is caramelized in concentrated syrup. The caramel aroma is mainly due to a group of cyclic alkylenolones, dihydrofuranones, and pyrones.
Caramel as food additive
Caramel is also used in flavorings and flavor enhancers for a wide range of foods, including caramels, cakes, and biscuits.
There are four classes of caramel used as food additives and they are defined by the reactant added to the carbohydrate during production.
*Plain caramel
*Caustic caramel
*Ammonia caramel
*Sulfite ammonia caramel
Caramel colorant must be compatible with food products in which they are used, which usually means the absence of flocculation and precipitation in the food.
Caramel is made up from the following food-grade carbohydrate: dextrose, invert sugar, lactose, malt syrup, molasses, starch hydrolysates, and fraction thereof and sucrose, by carefully controlled heat treatment. A large amount of commercial caramel is produced from liquid corn syrup or glucose syrup.
Caramel coloring is freely soluble in water and insoluble in most organic solvents. In concentrated form the colorant has a distinctive burned taste that is unnoticeable at the typical levels of use.
Caramel colors are the most widely used food coloring agents, contributing about 90% by weight of the total coloring agents supplied in the UK food industry. World-wide 80% has been quoted.
Caramelization is done in the industry with different catalysts to produce either flavor or color.
For flavor purposes, sucrose is caramelized in concentrated syrup. The caramel aroma is mainly due to a group of cyclic alkylenolones, dihydrofuranones, and pyrones.
Caramel as food additive
January 8, 2014
The use of fondant for cakes
There are two types of icing: butter cream and fondant. Buttercream is fluffy with a lightly sweet taste. On the other hand, fondant is the consistency of putty and therefore is very moldable, allowing to intricate designs.
Fondant is an icing comprised of confectioners; sugar and gelatin (among other ingredients). It is a good substitute for buttercream, especially for cakes that for one reason or another, may not be kept in a refrigerated case at all times.
In its most common incarnation for cakes, fondant is kneaded into the form of soft dough and rolled out to form a smooth covering that’s seals in freshness and adds a perfect looking, smooth, hard coating to cakes.
The fondant may develop tiny cracks or ‘elephant skin’ if too much time elapses. An undericing provides extra sweetness and clean, smooth surface. Powdered sugar or cornstarch can be used on the countertop to keep the fondant from sticking.
Fondant is often preferred for cakes appearing at outdoor events because it tends to hold up in hot or humid conditions.
For quality icings, a suitable flavor should be added to the fondant. For chocolate, the use of unsweetened chocolate or bloc chocolate is recommended; for coffee, a coffee extract; whilst for fruit flavors, concentrates or juices of the fresh fruit should be used.
The use of fondant for cakes
Fondant is an icing comprised of confectioners; sugar and gelatin (among other ingredients). It is a good substitute for buttercream, especially for cakes that for one reason or another, may not be kept in a refrigerated case at all times.
In its most common incarnation for cakes, fondant is kneaded into the form of soft dough and rolled out to form a smooth covering that’s seals in freshness and adds a perfect looking, smooth, hard coating to cakes.
The fondant may develop tiny cracks or ‘elephant skin’ if too much time elapses. An undericing provides extra sweetness and clean, smooth surface. Powdered sugar or cornstarch can be used on the countertop to keep the fondant from sticking.
Fondant is often preferred for cakes appearing at outdoor events because it tends to hold up in hot or humid conditions.
For quality icings, a suitable flavor should be added to the fondant. For chocolate, the use of unsweetened chocolate or bloc chocolate is recommended; for coffee, a coffee extract; whilst for fruit flavors, concentrates or juices of the fresh fruit should be used.
The use of fondant for cakes
September 25, 2012
Sugar solutions and syrup
When sugar is added to water, sugar solutions is formed which is homogenous solution. Sugars are soluble in water and readily form syrups. If waters is evaporated, crystals are formed.
When sugar concentrated into syrup, it resist bacteria and prevents fermentation. Concentrated sugar solutions – syrups are susceptible to molds, but molds are much slower to attack foods than are bacteria.
Sugars form molecular solutions due to hydrogen-bond interchange. When sugar is placed in water the water molecules form hydrogen bonds with the sugar molecules, thus hydrating them and removing them from the sugar crystals.
The solutions needs to be boiled in order to reach the desired sugar concentration to produce a sugar syrup.
As the sugar boiled water evaporates and the solution becomes saturated. When the saturated solution is called it became supersaturated and easily precipitates sugars.
The concentration of sugar solutions can be measured using a hydrometer, refractometer, or flowmeter.
The concentrations measured by these instruments is converted into a degrees brix value, which is the food industry’s standard of identifying the sugar concentration in syrups or liquids.
Each degree (1 °) of Brix is equal to a 1% concentration of sugar in solution when measured at 20 ° C.
Sugar solutions and syrup
When sugar concentrated into syrup, it resist bacteria and prevents fermentation. Concentrated sugar solutions – syrups are susceptible to molds, but molds are much slower to attack foods than are bacteria.
Sugars form molecular solutions due to hydrogen-bond interchange. When sugar is placed in water the water molecules form hydrogen bonds with the sugar molecules, thus hydrating them and removing them from the sugar crystals.
The solutions needs to be boiled in order to reach the desired sugar concentration to produce a sugar syrup.
As the sugar boiled water evaporates and the solution becomes saturated. When the saturated solution is called it became supersaturated and easily precipitates sugars.
The concentration of sugar solutions can be measured using a hydrometer, refractometer, or flowmeter.
The concentrations measured by these instruments is converted into a degrees brix value, which is the food industry’s standard of identifying the sugar concentration in syrups or liquids.
Each degree (1 °) of Brix is equal to a 1% concentration of sugar in solution when measured at 20 ° C.
Sugar solutions and syrup
March 10, 2012
Sweetness
The most obvious sensory property of sugars such as glucose fructose and sucrose is their sweetness, which varies depending on the specific sugar. There is no objective test for measuring the degree of sweetness.
Lactose or milk sugar is the least sweet, whereas all investigators agree that fructose is the most sweet sugar. Maltose is less sweet than glucose.
Sweetness helps mask or balance both sourness and bitterness from other ingredients. It has enhancing effect on perception of food aromas, perhaps by signaling the brain that the food is a good energy source and therefore deserves special attention.
Sweet-tasting compounds are characterized by a glycopene, which is able to bind to G-protein coupled trans-membrane receptors on the tongue.
According to the AH-B theory of sweetness this glycopene must contain a hydrogen bond donor (AH) and a Lewis base (B) separated by about 0.3 nanometers.
The AH-B units binds with a corresponding AH-B on the sweetness receptor to produce the sensation of sweetness. Sugar are used as sweeteners in cadies and many other food products.
This include boiled sweets, chocolates, toffees, bakery foods and cakes. Sugar provides flavor appeal to foods, and therefore is incorporated into many foods.
Sweetness
Lactose or milk sugar is the least sweet, whereas all investigators agree that fructose is the most sweet sugar. Maltose is less sweet than glucose.
Sweetness helps mask or balance both sourness and bitterness from other ingredients. It has enhancing effect on perception of food aromas, perhaps by signaling the brain that the food is a good energy source and therefore deserves special attention.
Sweet-tasting compounds are characterized by a glycopene, which is able to bind to G-protein coupled trans-membrane receptors on the tongue.
According to the AH-B theory of sweetness this glycopene must contain a hydrogen bond donor (AH) and a Lewis base (B) separated by about 0.3 nanometers.
The AH-B units binds with a corresponding AH-B on the sweetness receptor to produce the sensation of sweetness. Sugar are used as sweeteners in cadies and many other food products.
This include boiled sweets, chocolates, toffees, bakery foods and cakes. Sugar provides flavor appeal to foods, and therefore is incorporated into many foods.
Sweetness
February 15, 2012
Fructose in human diet
Fructose is a simple monosaccharide, the simplest sugar found in nature. Fructose is a six carbon sugar like glucose but it is a ketose sugar, not an aldose, because it contains a ketone group and not an aldehyde group.
Fructose is found naturally in fruits and honey. It is also derived from disaccharide sucrose. Apple contains about 8 grams of fructose, mean while medium banana 5 grams of fructose.
Fructose also present in invert sugar, a syrup made from sucrose and used extensively in the food industry.
It is widely used in processed and manufactured foods as a cheaper and healthy alternative to sugar especially in the form of corn syrup.
Fructose metabolism takes place mainly in the liver (75%) and to a lesser extent in the intestine and kidney (25%).
Sorbitol, also widely distributed in fruits and vegetables, is converted to fructose in the liver by sorbitol dehydrogenase. In recent years increased consumption of fructose exoaisally from sweetened beverages has been associated with an increased prevalence of:
Obesity
High cholesterol
Osteoporosis
Impaired immune system
Insulin resistance syndrome
Fructose is found naturally in fruits and honey. It is also derived from disaccharide sucrose. Apple contains about 8 grams of fructose, mean while medium banana 5 grams of fructose.
Fructose also present in invert sugar, a syrup made from sucrose and used extensively in the food industry.
It is widely used in processed and manufactured foods as a cheaper and healthy alternative to sugar especially in the form of corn syrup.
Fructose metabolism takes place mainly in the liver (75%) and to a lesser extent in the intestine and kidney (25%).
Sorbitol, also widely distributed in fruits and vegetables, is converted to fructose in the liver by sorbitol dehydrogenase. In recent years increased consumption of fructose exoaisally from sweetened beverages has been associated with an increased prevalence of:
Obesity
High cholesterol
Osteoporosis
Impaired immune system
Insulin resistance syndrome
Fructose in human diet
November 20, 2011
History of Sugar
History of Sugar
Sugarcane cultivation and sugar refining were practiced in India, Arabia and the Mediterranean in the Middle Ages, apparently spread from a point of origin in northeastern India though the Muslim world.
By the time of the discovery of the Americas in the late 15th century, sugarcane cultivation had already spread to the Canary Islands.
Columbus and other early explorers of the Caribbean recognized that the climate weather was ideal for sugarcane cultivation and Columbus brought the first sugarcane from the Canary Islands to Santo Domingo in 1493.
The first sugar mill in the Western Hemisphere was built there in 1509, and by 1511, sugarcane was being harvested in Cuba. It spread rapidly to the other islands and to Mexico and Brazil.
The estates granted to Hernan Cortes in Mexico after his conquest there in 1524 were largely devoted to sugarcane production.
The Africa slaves trade stimulated a need for products that the European could barter for slaves in Africa which included distilled spirits.
Rum could be made from molasses, one of the by products of the sugar refining process.
The sugar and rums became essential parts of the triangular trades that brought millions of Africans to the New World over the 300 years from about 1520 to about 1820. Since reducing the sugarcane to raw sugar and molasses required a heating process the island regions were soon denuded of firewood.
Planters would crush the sugarcane to separate the fibers in heavy roller mills usually powered by draft animals or slaves.
The crush cane would be rinsed repeatedly with water to extract the sugar juices. After the exhaustion of firewood, planters used the fibrous remains of the canes, called bagasse, as fuel for the crude boiling pits to crystallize the raw sugar.
By the 1600s, the refining process had been separated from the plantations. Raw sugar was shipped to refineries in Lisbon Marseilles, London and Amsterdam and later to New York and other cities in colonies North America.
The history traced that the industrialization to Europe and the northern American colonies to the place in the sugar induced triangular trade and the reduction of the Caribbean region to a plantation economy based on cheap labor to the same factor.
When France became isolated from its sugarcane colonies following the French revolution, the loss of Haiti (the French part of the island of Santo Domingo) to a slave-led revolution led the French to begin sugar beet production.
The process of refining sugar from beets had been develop in about 1750 by German chemist Andreas Margraf.
A small sugarcane plantation economy developed in Louisiana Territory between 1750 and 1800 and remained in place after the purchase of that territory by the United States in 1803.
History of Sugar
Sugarcane cultivation and sugar refining were practiced in India, Arabia and the Mediterranean in the Middle Ages, apparently spread from a point of origin in northeastern India though the Muslim world.
By the time of the discovery of the Americas in the late 15th century, sugarcane cultivation had already spread to the Canary Islands.
Columbus and other early explorers of the Caribbean recognized that the climate weather was ideal for sugarcane cultivation and Columbus brought the first sugarcane from the Canary Islands to Santo Domingo in 1493.
The first sugar mill in the Western Hemisphere was built there in 1509, and by 1511, sugarcane was being harvested in Cuba. It spread rapidly to the other islands and to Mexico and Brazil.
The estates granted to Hernan Cortes in Mexico after his conquest there in 1524 were largely devoted to sugarcane production.
The Africa slaves trade stimulated a need for products that the European could barter for slaves in Africa which included distilled spirits.
Rum could be made from molasses, one of the by products of the sugar refining process.
The sugar and rums became essential parts of the triangular trades that brought millions of Africans to the New World over the 300 years from about 1520 to about 1820. Since reducing the sugarcane to raw sugar and molasses required a heating process the island regions were soon denuded of firewood.
Planters would crush the sugarcane to separate the fibers in heavy roller mills usually powered by draft animals or slaves.
The crush cane would be rinsed repeatedly with water to extract the sugar juices. After the exhaustion of firewood, planters used the fibrous remains of the canes, called bagasse, as fuel for the crude boiling pits to crystallize the raw sugar.
By the 1600s, the refining process had been separated from the plantations. Raw sugar was shipped to refineries in Lisbon Marseilles, London and Amsterdam and later to New York and other cities in colonies North America.
The history traced that the industrialization to Europe and the northern American colonies to the place in the sugar induced triangular trade and the reduction of the Caribbean region to a plantation economy based on cheap labor to the same factor.
When France became isolated from its sugarcane colonies following the French revolution, the loss of Haiti (the French part of the island of Santo Domingo) to a slave-led revolution led the French to begin sugar beet production.
The process of refining sugar from beets had been develop in about 1750 by German chemist Andreas Margraf.
A small sugarcane plantation economy developed in Louisiana Territory between 1750 and 1800 and remained in place after the purchase of that territory by the United States in 1803.
History of Sugar
November 4, 2011
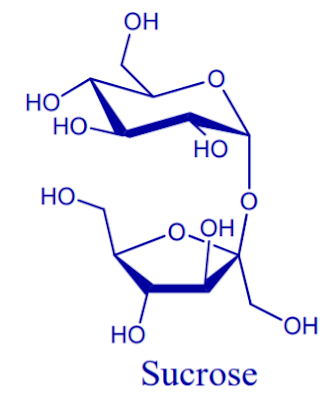
Sucrose
Three disaccharides are important in nutrition: maltose, sucrose and lactose. All three have glucose as one of their single sugars.
Sucrose is the most common disaccharide and it contains glucose and fructose joined together by an alpha-1,2-glyccosidic link.
Sucrose is the chemical name for what is commonly called white sugar, table sugar, granulated sugar or simply sugar. Sucrose provides some of the natural sweetness, of honey, maple syrup, fruits and vegetables.
The carbonyl groups of both the glucose and the fructose molecule are involved in the glycosidic bond; thus, the configuration of each monosaccharide become fixed.
Sucrose can be hydrolyzed to glucose and fructose by heat and acid or by the enzymes invertase or sucrose.
Sucrose is found in many plants and is especially abundant in sugar cane and sugar beets. These plants can be crushed to produce a juice that is recessed to make a brown liquid called molasses.
The equimolar mixture of glucose and the fructose produced in this way is called invert sugar.
Production of inverts sugar is important during the formation of candies and jellies, as inverts sugar prevents unwanted or excessive crystallization of sucrose.
Manufacturers use a refining process to extract sucrose from the juices of sugar cane or sugar beets. Full refining removes impurities white sugar and powdered sugar are so highly refined that they are virtually 100 percent sucrose.
Sucrose
Sucrose is the most common disaccharide and it contains glucose and fructose joined together by an alpha-1,2-glyccosidic link.
Sucrose is the chemical name for what is commonly called white sugar, table sugar, granulated sugar or simply sugar. Sucrose provides some of the natural sweetness, of honey, maple syrup, fruits and vegetables.
The carbonyl groups of both the glucose and the fructose molecule are involved in the glycosidic bond; thus, the configuration of each monosaccharide become fixed.
Sucrose can be hydrolyzed to glucose and fructose by heat and acid or by the enzymes invertase or sucrose.
Sucrose is found in many plants and is especially abundant in sugar cane and sugar beets. These plants can be crushed to produce a juice that is recessed to make a brown liquid called molasses.
The equimolar mixture of glucose and the fructose produced in this way is called invert sugar.
Production of inverts sugar is important during the formation of candies and jellies, as inverts sugar prevents unwanted or excessive crystallization of sucrose.
Manufacturers use a refining process to extract sucrose from the juices of sugar cane or sugar beets. Full refining removes impurities white sugar and powdered sugar are so highly refined that they are virtually 100 percent sucrose.
Sucrose
January 9, 2010
Carbohydrates
What is carbohydrates?
In order to carry out its day to day physiological functions and maintain a constant body temperature (due to invariably in a environment of changing temperatures), the body requires a constant source of energy.
Beyond it continuing maintenance needs for energy, the body periodically needs relatively larger amounts of energy to do work or to engage in other vigorious physical activites.
Human derives derive their energy mainly from carbohydtares (55-65%), although they can also utilise fats and proteins fo this purpose.
The carbohydrates are a class of chemical compounds that consists of carbon, oxygen and hydrogen.
The carbohydartes that are important in nutrition include the sugars, the starches, the dexrins and glycogen. Cellulose, pectin, and other carbohydrates are not important nutritonally.
Food Science
In order to carry out its day to day physiological functions and maintain a constant body temperature (due to invariably in a environment of changing temperatures), the body requires a constant source of energy.
Beyond it continuing maintenance needs for energy, the body periodically needs relatively larger amounts of energy to do work or to engage in other vigorious physical activites.
Human derives derive their energy mainly from carbohydtares (55-65%), although they can also utilise fats and proteins fo this purpose.
The carbohydrates are a class of chemical compounds that consists of carbon, oxygen and hydrogen.
The carbohydartes that are important in nutrition include the sugars, the starches, the dexrins and glycogen. Cellulose, pectin, and other carbohydrates are not important nutritonally.
Food Science
Subscribe to:
Posts (Atom)
The Most Popular Posts
-
Ash or mineral content is the portion of the food or any organic material that remains after it is burned at very high temperatures. The a...
-
Crude fat is the term used to refer to the crude mixture of fat-soluble material present in a sample. Crude fat also known as the ether ext...
-
Crude fiber is a measure of the quantity of indigestible cellulose, pentosans, lignin, and other components of this type in present foods. ...
-
Density is the weight of a substance per unit of volume, while specific gravity is the ratio between the density of the substance and that o...
-
Gelatinization occurs when starch granules are heated in a liquid. It is responsible for the thickening of food systems. The process is an i...